Pharmaceutical companies are always looking for new opportunities to improve offerings and deliver better products, but traditional processes and R&D methods no longer work as the industry faces significant digital transformation initiatives.
To remain competitive and relevant in the industry, enterprise pharma companies must modernize legacy applications by migrating to new cloud technologies, adopting emerging technologies like GenAI an RPA, and enabling end-users with better technology experiences. This gives a competitive advantage, provides better quality assurance, creates better products, maintains compliance, and continues to grow.
For most pharma companies, digital transformation is powered by advances in AI, process automation, and big data to improve decision-making and efficiency. But that starts with undergoing an organizational-wide pharma transformation.
Digital transformation in pharma is a significant investment — but all companies, regardless of industry, face digitalization challenges.
In this article, we’ll explore the impact of digital transformation on the pharmaceutical and life science industry, examples of digitalization in the industry, and key digital transformation challenges unique to pharma.
What are examples of digital transformation in the pharma industry?
- Smart quality with quality management systems (QMS)
- Digitization of regulatory approval and maintenance with regulatory information management (RIM) systems’
- Clinical trial digitization with e-clinical platforms
- DataOps to scale analytics and data projects
- Decisions based on AI and ML
- On-demand patient access and support programs
- Digital pills
- MLOps (machine learning operations) for pharmaceuticals
- Cloud decision platforms
- Smart drug development platforms
What Is Digital Transformation in Pharma?
Digital transformation in the pharma sector refers to companies updating legacy applications, systems, and processes with cloud applications and emerging technologies to optimize, improve, and revolutionize drug development, manufacturing, distribution, and patient care – as well as enterprise operational functions like sales, procurement, and people management.
Digital transformation initiatives in pharma focus on enhancing operational efficiency, driving innovation, providing better customer experiences, and improving the quality of products and services.
Research from Deloitte shows that digital transformation is expected to have a major impact on organizational strategies in 2025. About 60% of executives cited gen AI or digital transformation as key emerging trends they closely monitor. Moreover, nearly 60% of executives plan to increase gen AI investments across the value chain, suggesting that companies are moving beyond initial pilot projects.
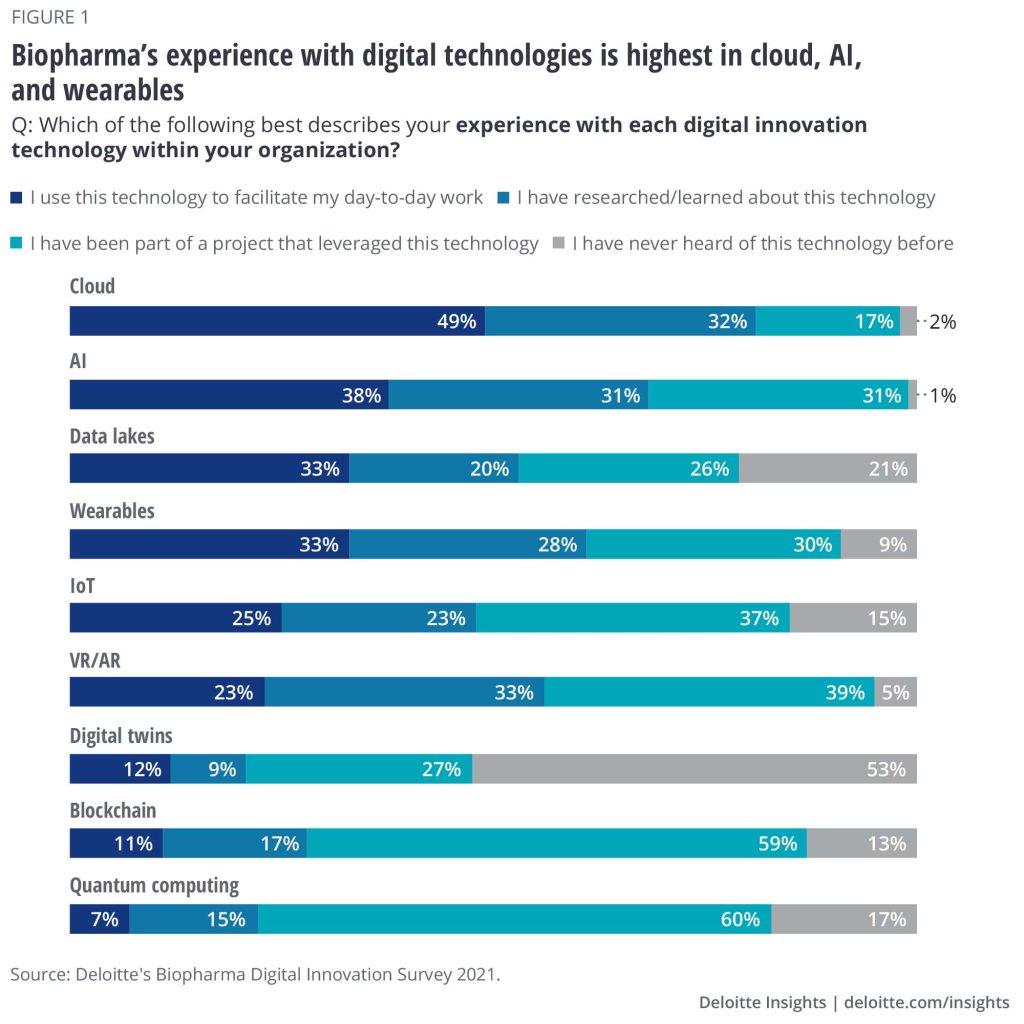
Examples of Digital Transformation in Pharma
Here are a few examples of digital transformation initiatives contextual to the pharma industry and life science companies.
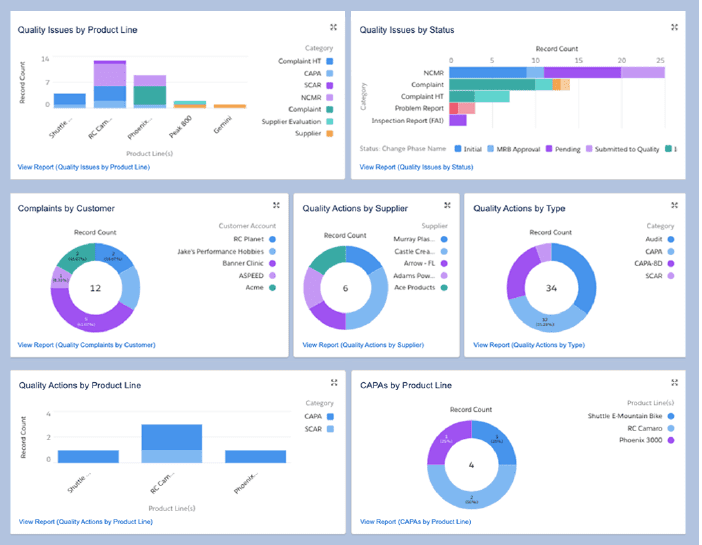
1. Smart quality with quality management systems (QMS)
Quality management systems (QMS) use data analytics and real-time monitoring to identify quality issues before they spread quickly. Quickly detecting faulty products — especially in the case of something as sensitive as pharmaceuticals — not only saves money and maintains a high brand standard, but can also save lives.
A QMS provides oversight into several functions related to maintaining quality standards, including:
- Q/A and maintenance of all pharmaceuticals produced and shipped
- Risk assessment and regulatory compliance
- Data migration, management, and reporting
- Incident and complaint management
- Training management
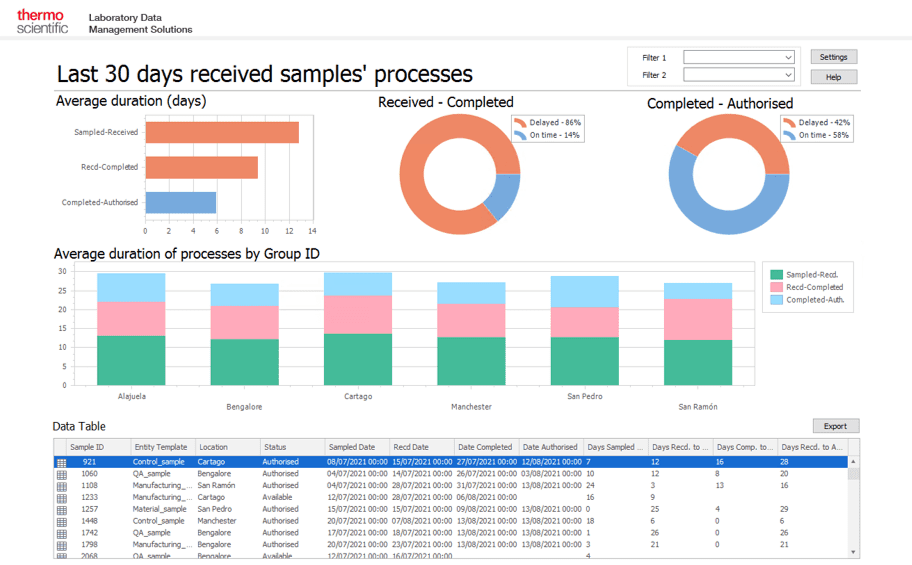
2. Digitization of regulatory approval and maintenance with regulatory information management (RIM) systems
A regulatory information management (RIM) system centralizes and manages all regulatory data, making it easier to ensure all processes and systems align with compliance requirements. RIM systems digitize the approvals process, reducing the risk of human error and minimizing complexity.
In pharma, a RIM system supports the following processes:
- Product registration management
- Regulatory requirements and submissions management
- Product detail, information, and label development and management
- Submission planning, management, and storage
3. Clinical trial digitization with e-clinical platforms
E-clinical platforms can improve the speed and quality of trials while managing costs and resources. It streamlines the collection, analysis, and reporting of clinical trial data, allowing for faster decision-making with lower costs. E-clinical platforms also provide access to real-time data, allowing for in-the-moment changes or adjustments.
Introducing e-clinical platforms to trials can also support pharma companies with:
- Integrating multiple e-clinical point solutions into a single platform.
- Planning, forecasting, and assessing clinical trials.
- Capturing, storing, and managing data.
- Collecting participant information and consent.
- Generating trial analytics reports.
Related Resources
4. DataOps to scale analytics and data projects
DataOps can improve the integration and management of data, creating a more consistent data environment. In the pharma industry, this means faster insights and improved decision-making that lead to safer and more effective care.
DataOps can support pharma digital transformation by:
- Extracting more value from data
- Enabling strategic innovation
- Promoting a data-driven go-to-market strategy
- Developing clear milestones and performance metrics
5. Decisions based on AI and ML
Artificial intelligence (AI) and machine learning (ML) can analyze massive datasets in minutes, making it easier to make critical decisions like identifying drug candidates, predicting and forecasting outcomes, and optimizing logistics. Using AI and ML to improve pharma decision-making means better patient care and more positive care outcomes.
AI and ML in pharma can make the following processes more efficient:
- Running trial simulations and tests to ensure participant safety
- Identifying supply chain shortages or challenges before they arise
- Predicting patient reactions
- Selecting trial participants to match overall goals
6. On-demand patient access and support programs
Between running trials and providing care via pharmaceuticals, companies must provide those under their care with access to reliable support. Digital tools can give 24/7 access to healthcare info and telemedicine support to ensure their safety and provide the best possible care.
Providing on-demand patient support can:
- Build trust with patients and trial participants
- Enhance patient engagement and improve treatment plan success rates
- Catch and address patient problems early
7. Digital pills
Digital pills contain an ingestible sensor that allows healthcare providers to monitor patient care and medication efficiency. The pill sensor collects and transmits data that can be used to adjust care plans more effectively.
The use of digital pills can also:
- Reduce patient healthcare costs
- Provide more accurate patient data to improve care decision-making further
- Ensure more personalized patient care opportunities
8. MLOps for pharmaceuticals
MLOps streamlines and automates AI model development in pharma, turning one-off experiments into repeatable processes. This factory-like approach helps companies quickly build and deploy AI models for tasks like drug discovery, trial optimization, and patient outcome prediction.
In fact, McKinsey research indicates that companies adopting this approach are much more likely to realize scale and value from their AI investments, with some adding as much as 20% to their earnings before interest and taxes.
MLOps helps pharma companies:
- Standardize AI development across departments
- Reuse tested code components for faster deployment
- Monitor model performance automatically
- Achieve compliance and reduce technical risks
- Scale successful AI projects
9. Cloud decision platforms
Cloud platforms connect AI, automation, and real-time data to help teams make faster, better decisions. These systems pull information from multiple sources to support everything from drug development to sales operations.
Cloud platforms deliver:
- On-demand access to critical data
- Automated workflow management
- Real-time analytics dashboards
- Flexible scaling of computing resources
- Integrated compliance monitoring
However, pharmaceutical companies will only achieve their intended outcomes from cloud technologies if they’re properly adopted by end-user employees. This requires contextual end-user training and support, as well as continuing to refine pharmaceutical business processes to remove confusion and friction.
With Whatfix, pharma companies can make their software investment works for their use cases and users, not the other way around. With user-centric software, time-to-completion for tasks and processes is optimized, fewer errors occur, and employees utilize more advanced features that drive business outcomes and help organizations realize technology ROI.
10. Smart drug development platforms
These platforms combine AI algorithms and real-world data to speed up drug discovery and development. Companies like BioNTech use AI-powered platforms to design and test immunotherapies faster and predict which drug candidates have the highest chance of success.
These platforms provide:
- Automated screening and design
- Predictive modeling for drug outcomes
- Real-time clinical trial data analysis
- Integration with lab automation systems
- Risk assessment and safety monitoring
Digital Maturity of the Pharma and Life Science Industry
Pharma companies are rapidly adopting digital tools, with 68% of executives expecting revenue growth from digital investments in 2025. Companies now spend up to 20% of their earnings on digital programs, focusing on drug development automation, clinical trials, and patient care.
This shift matters because it transforms how drugs reach patients. AI speeds up research, cuts development costs, and helps predict which treatments will work best. Companies that master these tools bring medicines to market faster and cheaper, while those that fall behind risk losing their competitive edge.
Digitization Challenges in Pharma
While digital transformation initiatives can dramatically improve the pharma industry, digitalization is a major project that requires dedicated project scoping, budget, resources, migration, implementation, deployment, and ongoing support.
There are also digital transformation challenges that are contextual the the pharma industry. Here are some of the biggest hurdles pharma companies face when undergoing digitization.
1. Patient data and privacy
Digitization often involves the use of massive amounts of data—and in pharma’s case, that data is linked to patients and their sensitive care information. Dealing with sensitive patient information can raise concerns about privacy and data security.
Companies need to ensure they’re complying with regulations like GDPR and HIPAA. Failure to comply leads to steep fines, and a breach of sensitive patient data can lead to a lack of trust and a negative perception of the company.
2. Upskilling employees with new digital skills
New digital operations and processes mean employees must know how to use next-generation tools and technologies, such as QMS and RIM systems, among many other new application types.
Pharma employees must be provided proper onboarding and upskill training to do their jobs efficiently with these new digital applications and processes. Without proper training, pharma companies will have employees who can’t use these new technologies – leading to poor end-user performance and productivity, failure to achieve digital transformation ROI, and much more that impacts pharma companies’ bottom line, output, customer experience, and revenue,
Introducing employees to new digital skills may lead to resistance and pushback. Companies must implement effective change management initiatives to ensure they get the most out of their digital transformation.
3. Technology-related concerns
Introducing new technology requires properly integrating tools across the entire organization and building a compatible IT infrastructure to match how your departments and teams work. This means integrating and migrating all critical data to the cloud or building a hybrid storage environment, as well as breaking down data silos and enabling your pharma company’s various departments with data warehouses that bring together all this data.
Technology changes can be complex, so you’ll need to build a team capable of handling these problems or hire an outside digital transformation consulting company. Your IT team should be proactive in preventing significant issues like application downtime or lost data – and should be able to provide help desk and service support to team members who need assistance using these new pharma-specific applications.
Pharma IT teams can provide contextual, real-time support to employees through:
- Help desk systems that allow employees to submit support tickets to IT on their specific issue(s).
- IT self-service portals that enable employees with a curated dashboard of self-help resources similar to a knowledge base on answers to common IT-related questions.
Digital adoption platform (DAP) that enables employees with in-app guided flows, nudges, and smart tips – as well as an embedded Self Help wiki that aggregates your internal documentation, IT portal, SOPs, videos, training resources, third-party links, and more into your digital pharma applications and workflows.
CASE STUDY
Ferring Pharmaceutical uses Whatfix to support employees within its Icertis contract management workflows, maximizing the value from its CLM transformation and investment. Facing regulatory and compliance contracting requirements specific to the pharma industry, Ferring enabled its Icertis end-users with in-app guidance that reduced time-to-proficiency for new users, resolved friction points in its complex contract workflows, and drove Icertis adoption.
“When they showed Whatfix to me, I approached our CLM steering committee and said that we cannot successfully implement the CLM solution unless we have Whatfix,” said Sheila Dusseau, Head of Global Legal Operations at Ferring.
4. FDA and drug regulations
Even while undergoing a digital transformation, pharma companies must prioritize meeting FDA requirements and other drug regulations. These rules are constantly evolving, but they’re there to maintain patient safety and ensure legal compliance.
While determining what technological developments to implement, look for opportunities to make meeting regulations easier. Put patient care at the center of all the decisions your team makes.
5. Ethics
It’s exciting to advance pharma technology as much as possible, but ethics play a crucial role in how technology is used. This is especially true when working with patient data. Regarding research and development, patients’ and trial participants’ safety always needs to come first.
Pharma Technology Clicks Better With Whatfix
Digital transformation is challenging, costly, resource-intensive, and has lengthy implementation and migration cycles. Accelerate your pharma digital transformation by enabling your employees with software, tasks, and workflows designed for them. With Whatfix, organizations can take a multi-step approach to value realization of pharma transformation with:
- Whatfix DAP provides a no-code editor to create onscreen overlays and in-app guidance like Task Lists, Flows, Smart Tips, and Self Help that support employees in the flow of work, guiding them through core tasks to help drive process adoption, ensure compliance, and act as guardrails for workflow governance.
- Whatfix Mirror enables L&D managers to easily create replica sandbox environments of enterprise applications, providing employees with a risk-free, interactive application training environment.
- Whatfix Product Analytics provides a no-code event track and user behavior solution to understand how employees are engaging with technologies and workflows, identify areas of user friction or process inefficiencies, map optimal end-user flows for different departments and roles, and drive overall adoption of pharma enterprise systems and technologies like QMS, RIM, Procurement, Contract Management, and more.